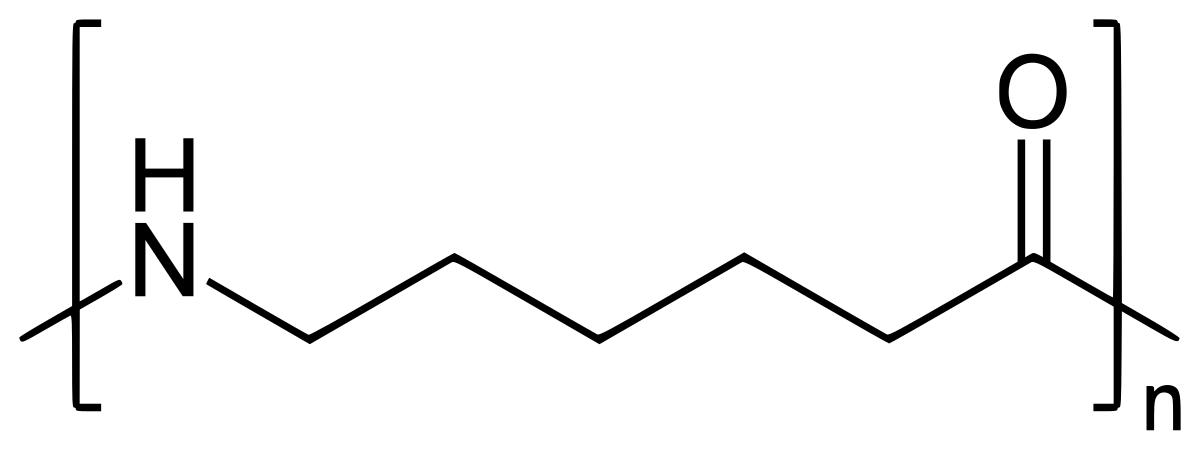
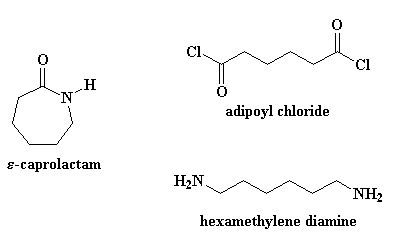
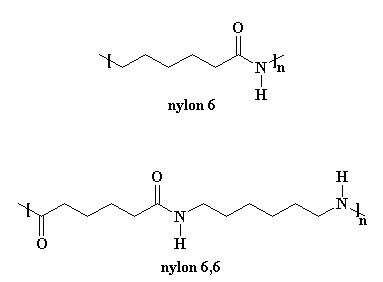
The monomer units of nylon-66 are hexamethylenediamine and adipic acid. For nylons made from A-AB-B monomer systems the two numbers tell you how many carbon atoms are in the diamine monomer and how many carbons are in the diacid or diacid chloride monomer.
Initiator Ph I I Ph Ph I Ph Alternating Copolymer Random Copolymer Monomer Unit 1 Monomer Unit 2 Cl Cl Cl n ClCl Saran N H O H N O N H O O H N 6 carbon diamine unit 6 carbon dicarbonyl unit Nylon 66.
. Nylon 6 is formed by the ring-opening polymerization of caprolactam. The diagram below represents a cell organelle. Nylon 6 or polycaprolactam is a polymer developed by Paul Schlack at IG Farben to reproduce the properties of nylon 66 without violating the patent on its production.
Nylon is a generic term for the polyamide type product of polymeriation of a diamine and a dicarboxylic acid. Draw the structure of nylon 46. Ii Propene is structural formula CH 3 - CH CH 2 of polypropene.
Draw the structure of the monomer for each of the following polymers. I Nylon 6 or polycaprolactam is a polymer formed by ring-opening polymerization of caprolactam as shown in the above figure. Draw the skeletal formula of each monomer used to make nylon 610.
Draw the structures of the monomers of the following polymers. An example of this type is nylon 6 the structure of which is shown in Fig. Nylon 6-12 Polyhexamethylene dodecanediamide STRUCTURE BASED NAME.
What is the percent yield of polymer that you obtained in the laboratory experiment. B State the function of. Advertisement Remove all ads.
Thus the monomer of nylon 6 is caprolactam. The monomeric repeat unit of Nylon-6 6 polymer is N H C H 2 6 N H C O C H 2 4 C O which is derived from the two monomers hexamethylene diamine N H 2 C H 2 6 N H 2 and adipic acid H O C O C H 2 4 C O O H as shown in the figure. Nylon 6 6 is a condensation polymer whose structure is as follows.
Draw the structures of the monomers in nylon 6 6. Phenol and formaldehyde Condensation polymer. I Nylon 6 ii.
When a single monomer is polymerized the product is made of chains whose repeat unit corresponds to the monomer. All India 2010 Bakelite Nylon-6 Ans. I NH - CH 2 5 - CO which is derived from Caprolactam.
Nylon 6 is very strong and elastic in nature. Hence if a nylon is named nylon 6 you know that it is made from an A-B monomer and that A-B monomer has six carbon atoms. There are two monomers in this case.
Draw the structures of monomers of the following polymers. In the particular case of Nylon 6 patented by. Kavungya answered the question on May 23 2019 at 1340.
This polymer also has the six-carbon diamine unit but the dicarbonyl unit contains ten carbon atoms. Solution 1 Show Solution. Nylon-6 is a polymer constructed by a ring-opening polymerization unlike most nylon polymers which are synthesized using condensation polymerization.
Nylon 46 is a commonly used industrial nylon variant that can withstand higher temperatures than. Around the same time Kohei Hoshino at Toray also succeeded in synthesizing nylon 6 It is a semicrystalline polyamideUnlike most other nylons nylon 6 is not a condensation polymer but instead is. A Name the part labelled B.
Nylon is the general name for a family of polyamides. Draw the skeletal formula of an alternative monomer to the dicarboxylic acid drawn in part a to make nylon 610. Use the skeletal formulae in part a to draw an equation showing the condensation polymerisation to make nylon 610 from a diamine and a dicarboxylic acid.
If two different monomers are polymerized the result most often is a chain whose repeat unit corresponds to the two different monomers arranged alternately. The monomeric repeating unit of nylon -6 is. Frequently encountered synthetic nylon polymer is Nylon 610.
Write the structures of the monomers used for getting the following polymers. Total 2 marks 2. C O C O N H CH 2 8 N H CH 2 6 C O C O N H CH 2 8 N H CH 2 6 Draw the structures of two monomers that could be used to make this nylon.
Use the structure of Nylon-6 to deduce and draw its cyclic monomer. Nylon 6 is used in manufacturing of carpets hosiery seat. Synthetic polyamides such as nylon contain the same link as polypeptides.
Ii Polypropene also known as Polypropylene PP is a thermoplastic polymer made by addition polymerization of the monomer propene it is rugged and unusually resistant to many chemical solvents bases and acids. It is very tough easy to wash and dye and is resistant to abrasion. Draw the structure of the monomer for each of the following polymers.
Advertisement Remove all ads. The amide bond of the caprolactam molecule is broken during the polymerisation reaction. Delhi 2012 i Nylon-6 ii Polypropene Ans.
Write the Names and Structures of the Monomers of the Following Polymers. I Bakelite ii Nylon - 6 asked Dec 7 2019 in Chemistry by ParveenSharma 950k points class-12. Draw the structure of the monomer for each of the following polymers.
A short section of a nylon polymer is shown below. Draw the structures of the monomers in nylon 6 6. Polymer Name of Monomers Structure of Monomers i Nylon-66 Hexamethylenediamine and adipic acid ii PHBV 3-Hydroxypentanoic acid and 3.
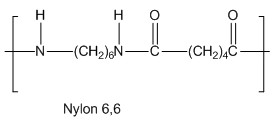
Write The Structures Of Monomers Used To Obtain The Following Polymers A Natural Rubber B Pvc C Nylon 6 6 Chemistry Topperlearning Com 3t7venyy
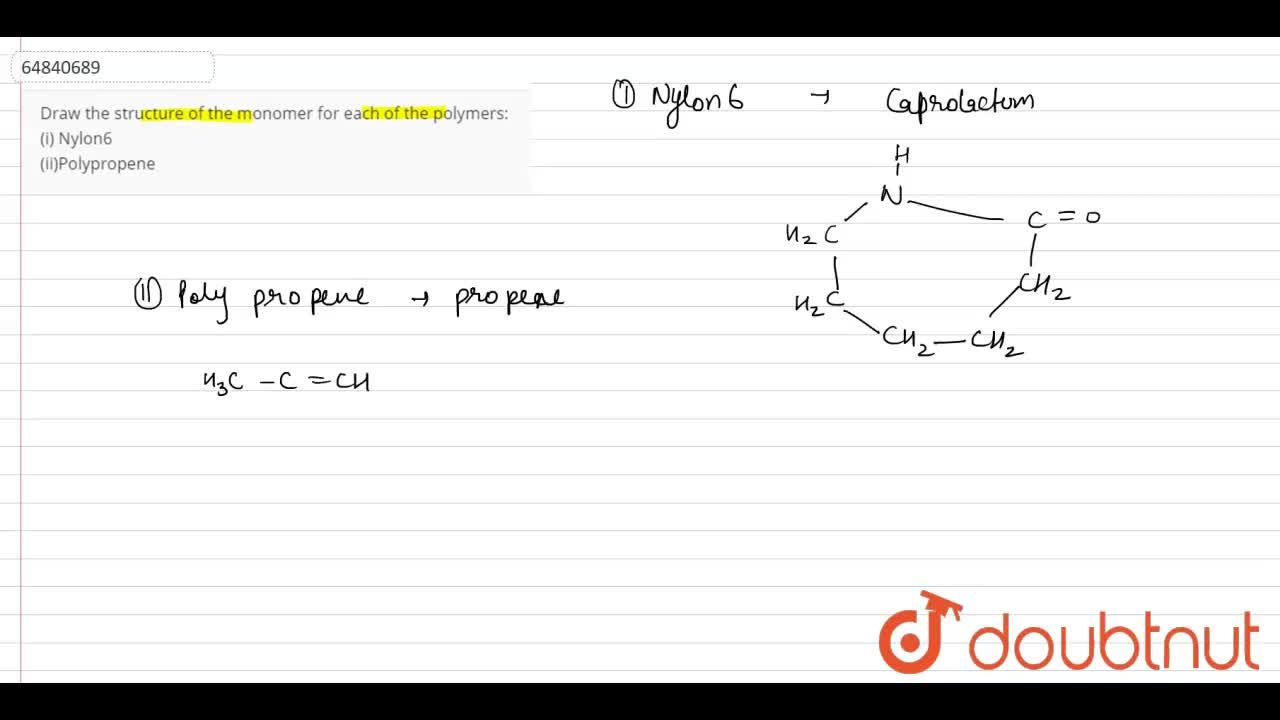
Draw The Structure Of The Monomer For Each Of The Polymers I Nylon6 Ii Polypropene
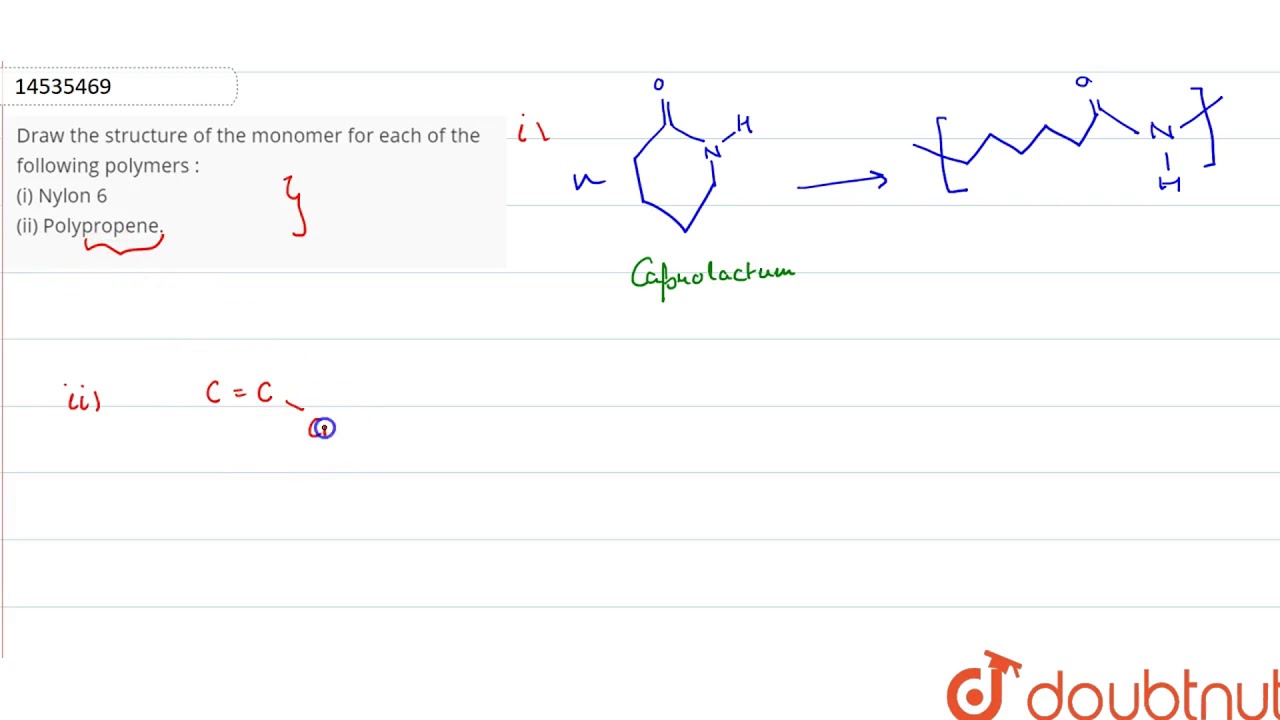
Draw The Structure Of The Monomer For Each Of The Following Polymers I Nylon 6 Ii Polypropene Youtube
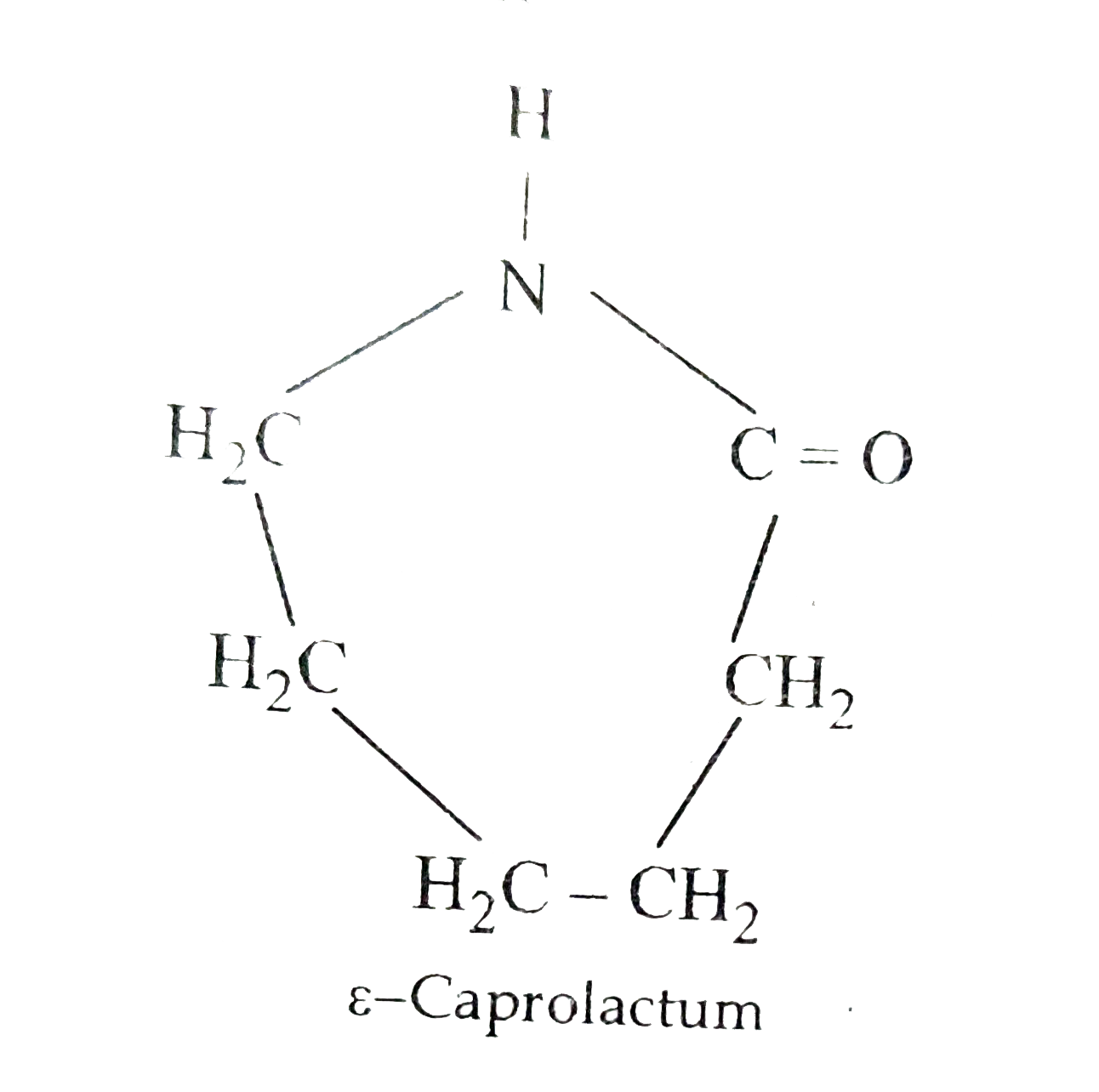
Draw The Structure Of The Monomer For Each Of The Polymers I Nylon6 Ii Polypropene
Draw The Structure Of The Monomer For Each Of The Following Polymers I Nylon 6 Sarthaks Econnect Largest Online Education Community

Write The Names And Structures Of The Monomers Of The Following Polymers Nylon 6 Chemistry Shaalaa Com
0 comments
Post a Comment